454 GS Junior系统将增加测序读长及推出新自动化方案
454 GS Junior测序仪将在2012-2013年增加测序读长及推出新自动化方案
454 GS Junior测序仪最近推出了其Phase B的 一系列改进,包括新版本的测序软件v2.7,相比以前v2.5软件,对于图像处理有明显提升,可以带来更加稳定的测序结果,尤其是扩增子的测序。同时,454公司也将在2012年-2013年间陆续推出一系列提高测序性能以及自动化工作流程的GS Junior升级方案。GS Junior系统在传染病,癌症,环境微生物及农业研究方面都有广泛应用。1
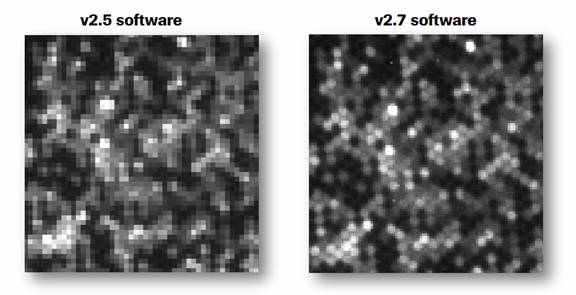
新的测序软件v2.7提高了测序稳定性以及测序效率,除了在图像处理方面的提升外也在GS De Novo Assembler, GS Reference Mapper以及GS Amplicon Variant Analyzer软件上增加了一些新的功能。在实验方面,paired end测序(可以获得跨越3 kb, 8 kb或者20 kb区域的两段序列)推出了使用GS FLX Titanium Rapid Library Preparation Kits的文库制备方案,加快建库速度,同时可以为样本添加特异性标签进行混合测序。
此次特别宣布了关于GS Junior系统的一系列后续升级方案,包括增加测序读长,自动化文库制备和emPCR工作流程的计划。这些方面的提升将帮助原先使用传统Sanger测序方法的研究人员方便转移到454测序平台上,无论是基础研究还是转化医学领域的研究人员,能使用GS Junior系统。这些改进预计最快在今年的晚些时候或者明年年初正式推出。
454 Life Sciences总裁Thomas Schinecker先生说道:“我们很高兴看到GS Junior系统在全球得到推广,同时也很兴奋地向大家宣布我们为不断提高其性能、简便操作所做的各种努力及规划。这些改进将使得454的长度长平台更加人性化,使得更多传统测序使用者可以转移到二代测序平台上。”
1 了解更多GS Junior相关文献,请浏览 http:///publications.
Roche Launches Software Upgrade for GS Junior System, Announces Long Read Improvements and Workflow Automation for 2012/2013
Apr 16, 2012 - fuchsc8
Roche announced today the immediate availability of a new software upgrade and further upcoming sequencing performance and workflow improvements to the company’s 454 GS Junior System. Now a widely published benchtop next-generation sequencing platform, the GS Junior System is rapidly advancing research worldwide in areas of infectious disease, cancer, environmental microbiology and agriculture1.
The new software package (v2.7) improves sequencing consistency and robustness and also includes functionality improvements to the GS De NovoAssembler, GS Reference Mapper, and GS Amplicon Variant Analyzer Software. The company also announced the immediate availability of new protocols for paired end sequencing (3 kb, 8 kb, 20 kb span) using the GS FLX Titanium Rapid Library Preparation Kits. The protocols enable simple sample multiplexing for paired end projects and reduce overall prep time.
In addition, the company has announced upcoming improvements to the GS Junior System, which include extension to sequencing read lengths and automation of the library preparation and emPCR workflow. The improvements will further ease the transition of projects from traditional Sanger capillary sequencing to 454 Sequencing Systems in basic and translational research laboratories around the world. The planned improvements will first become available in late 2012 and during 2013.
“We are pleased by the adoption of the GS Junior System worldwide and are excited to announce our efforts to continuously improve performance and ease of use of the platform,” said Thomas Schinecker, President of 454 Life Sciences, a Roche Company. “These improvements make our long read sequencing solution more user friendly and enable customers to convert projects from Sanger to next-generation sequencing more easily.”
- 西藏药检所成功引进Phenom XL大仓室智能扫描电镜
- 易科泰FMT150藻类培养与监测系统落户天津大学
- 因美纳推出全新空间转录组学技术
- 2025瑞孚迪全球销售启动大会在拉斯维加斯圆满举行
- 国货当自强!微纯国产替代色谱图有奖征集活动开始啦
- 赛恩深度参与电子测量仪器产业及标准化研究报告编制
- 真迈生物再次获批“十四五”国家重点研发计划
- 科圣科技拿下Diagnostic Biochips中国区总代理
- 开年上新:尚恩生物细胞转染服务正式上线啦!
- 科德角国际获北京市专精特新与创新型中小企业认证
- 天隆联合西安交大共同推进的国家医学中心正式启动
- 富勒Fuller Laboratories加入兽医诊断领导者VMRD
- 华中科技大学与泰渡生物共建蛋白纯化示范实验室
- 棱镜泰克携手许川教授团队研制的新款流式细胞仪获证
- 华大智造副总裁中国区总经理彭欢欢一行到访伯豪生物
- 鼎昊源为北京某口腔医院供货114台离心机设备
- 莱比信诚聘区域销售经理
- 莱比信亚太区总裁张鹏专访——打破困境,出海全球
- 赫西离心机产品亮相第十二届慕尼黑上海分析生化展
- 2024 analytica盛况直击,Eppendorf科技之旅精彩启航
- 奥豪斯邀请您参加2024BTE第8届国际生物技术大会
- 鼎昊源新品:NX-1R Pro高速微量冷冻离心机闪亮登场
- 卢湘仪数百台TD5M离心机出口巴西拓展海外市场
- 莱比信邀您共赴2024中国材料大会
- 德国Herolab携多款设备亮相CPHI&PMEC China2024
- 德国Herolab邀您免门票参观CPHI & PMEC China 2024
- 赫西参与起草的实验室离心机行业国家标准完成修订
- 解密瑞沃德M416R高速冷冻离心机研发故事,可申请试用
- 奥豪斯新款台式冷冻离心机FC5720R、FC5830R上市
- 湘仪二十三周年厂庆:风雨兼程,与君同行






